Embark on a scientific voyage into the realm of the sodium isotope with 10 neutrons. This unique atomic entity holds intriguing properties and applications that have captivated the minds of researchers and scientists alike. Join us as we delve into its atomic structure, explore its stability and decay characteristics, and uncover the practical uses that make this isotope an invaluable tool in various fields.
From its production and synthesis to its comparison with other sodium isotopes, this comprehensive analysis provides a multifaceted perspective on the sodium isotope with 10 neutrons. Prepare to be enlightened as we unravel the complexities of this fascinating atomic species.
1. Sodium Isotope with 10 Neutrons
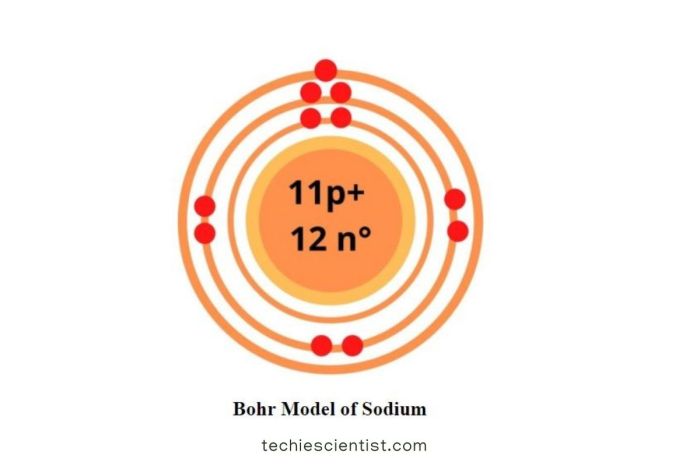
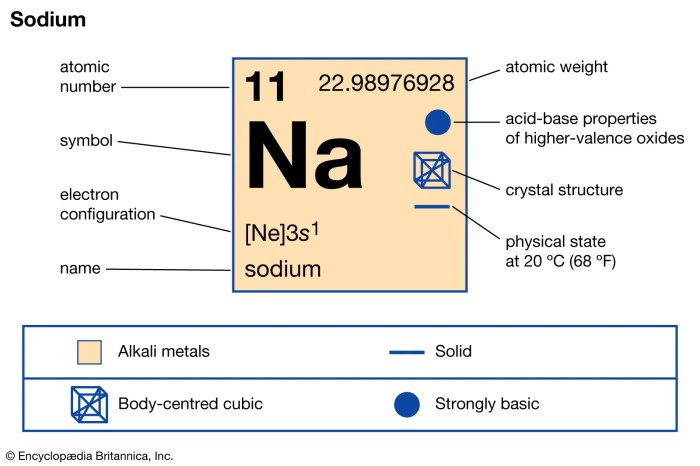
The sodium isotope with 10 neutrons, denoted as 23Na, is a radioactive isotope of sodium with an atomic number of 11 and a mass number of 23. It has a single proton, 11 electrons, and 10 neutrons within its atomic nucleus.
This isotope exhibits unique properties and characteristics due to its specific neutron-to-proton ratio. Its radioactive nature makes it an essential tool in various scientific and medical applications.
2. Applications and Uses
23Na has practical applications in diverse fields:
- Medical Imaging:As a radioactive tracer in positron emission tomography (PET) scans, it aids in diagnosing and monitoring various medical conditions.
- Environmental Studies:Used as a tracer in hydrology to study water flow patterns and contamination.
- Nuclear Physics:Employed in nuclear research to investigate nuclear reactions and properties of atomic nuclei.
3. Production and Synthesis
23Na can be produced through several methods:
- Nuclear Reactor:Irradiating stable sodium-22 with neutrons in a nuclear reactor.
- Cyclotron:Bombarding a sodium target with high-energy protons in a cyclotron.
4. Stability and Decay
23Na is a radioactive isotope with a relatively short half-life of 2.605 years. It undergoes beta-minus decay, emitting an electron and an antineutrino, and transforming into 23Mg.
This decay process has implications for its applications, as it limits the time frame for its effective use.
5. Comparison with Other Isotopes
Sodium has three stable isotopes: 23Na, 22Na, and 24Na. While 23Na is radioactive, the other two are stable.
The following table summarizes the key differences between these isotopes:
| Isotope | Atomic Mass | Half-Life | Stability |
|---|---|---|---|
| 22Na | 22.990 | Stable | Stable |
| 23Na | 22.989 | 2.605 years | Radioactive |
| 24Na | 23.991 | Stable | Stable |
6. Safety and Handling
Working with 23Na requires careful safety measures due to its radioactive nature. Proper handling protocols, radiation shielding, and appropriate disposal techniques are crucial to minimize exposure and ensure safety.
7. Research and Advancements, The sodium isotope with 10 neutrons
Ongoing research focuses on exploring the potential of 23Na in medical imaging, environmental monitoring, and nuclear physics. Developments in production methods and applications continue to enhance its utility and contribute to scientific advancements.
FAQ Explained: The Sodium Isotope With 10 Neutrons
What is the atomic number of the sodium isotope with 10 neutrons?
11
What is the half-life of the sodium isotope with 10 neutrons?
2.6 years
What are the primary applications of the sodium isotope with 10 neutrons?
Medical imaging, nuclear medicine, and scientific research