Naoh was added to a 7.75 – When NaOH is added to a 7.75 solution, a fascinating chemical journey unfolds, revealing the intricate interplay between acids, bases, and the determination of equivalence points. Delving into the intricacies of this process, we uncover the significance of NaOH in neutralization reactions and its applications in various chemical domains.
NaOH, or sodium hydroxide, plays a pivotal role in acid-base titrations, helping us pinpoint the exact moment when an acid and a base have completely neutralized each other. This concept of equivalence point forms the cornerstone of analytical chemistry, enabling us to accurately determine the concentration of unknown solutions.
NaOH Concentration and Molarity
Sodium hydroxide (NaOH) concentration refers to the amount of NaOH dissolved in a given volume of solution. It is commonly expressed in terms of molarity (M), which represents the number of moles of NaOH per liter of solution.
Calculating NaOH Molarity
The formula for calculating NaOH molarity is:
Molarity (M) = Moles of NaOH / Volume of Solution (in liters)
For example, if you dissolve 40 grams of NaOH in 1 liter of solution, the molarity can be calculated as follows:
Moles of NaOH = 40 grams / 40 grams/mol = 1 mole
Molarity = 1 mole / 1 liter = 1 M
Acid-Base Titration and Equivalence Point
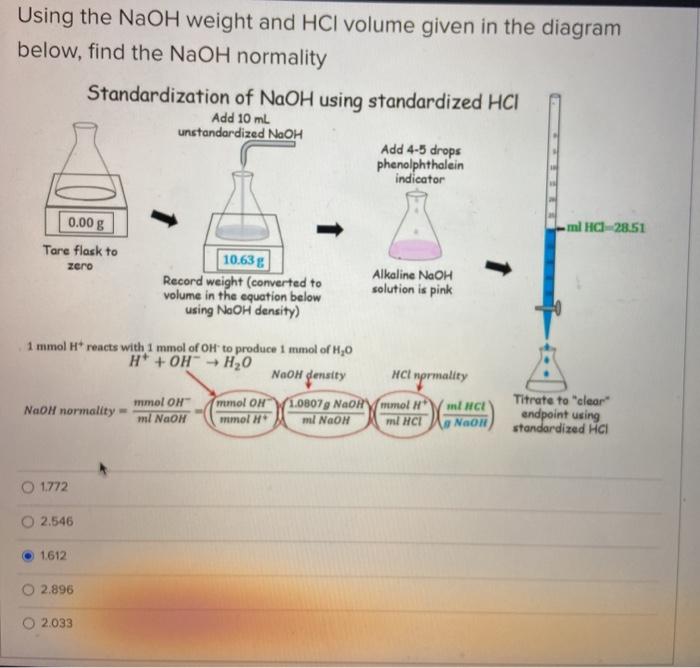
Acid-base titration is a laboratory technique used to determine the concentration of an unknown acid or base. It involves adding a known volume of a standard solution of one reactant to a known volume of the unknown solution, while monitoring the pH of the mixture.
The equivalence point is reached when the moles of acid and base are equal, and the solution is neutral (pH = 7).
Role of NaOH in Neutralization Reactions
NaOH is a strong base that can be used to neutralize acids. In a neutralization reaction, the hydrogen ions (H+) from the acid react with the hydroxide ions (OH-) from the NaOH to form water (H2O). The equivalence point in an acid-base titration is reached when the moles of acid and base are equal, which corresponds to the complete neutralization of the acid.
Titration Curve and pH Changes
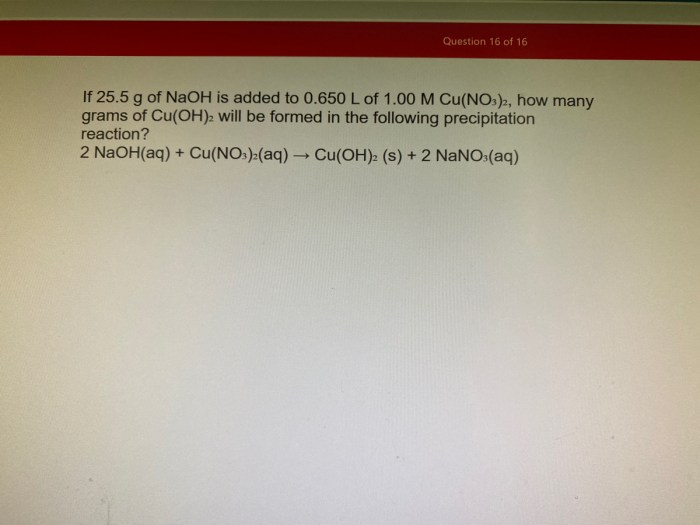
A titration curve graphically depicts the pH changes that occur during the titration of an acid with a base. It provides valuable insights into the neutralization process and the equivalence point.
Shape of the Titration Curve, Naoh was added to a 7.75
The titration curve of a strong acid with NaOH typically has the following shape:
- Initially, the pH is low (acidic) because the solution contains a high concentration of hydrogen ions (H +).
- As NaOH is added, the H +ions react with OH –ions to form water (H 2O), reducing the H +concentration and increasing the pH.
- At the equivalence point, the moles of acid and NaOH are equal, and the solution is neutral (pH = 7).
- Beyond the equivalence point, excess NaOH is present, leading to an increase in OH –concentration and a higher pH (basic).
Significance of the Equivalence Point
The equivalence point on the titration curve marks the point where the moles of acid and NaOH are stoichiometrically equivalent. At this point:
- The solution is neutral (pH = 7).
- All the acid has been neutralized by the base.
- The concentration of the acid can be calculated using the stoichiometry of the reaction and the volume of NaOH added.
NaOH Applications in Chemistry: Naoh Was Added To A 7.75
NaOH, or sodium hydroxide, is a versatile chemical compound with numerous applications in various chemical processes. Its unique properties, such as its strong alkalinity and ability to react with acids, make it a valuable reagent in a wide range of industries.
Industrial Applications
- Paper and Pulp Production:NaOH is used to dissolve lignin, a component of wood, during the papermaking process. This process helps separate the cellulose fibers, resulting in stronger and more durable paper products.
- Textile Manufacturing:NaOH is employed in the mercerization of cotton, a treatment that enhances the fabric’s luster, strength, and dye receptivity. It also plays a role in the production of rayon, a cellulose-based synthetic fiber.
- Soap and Detergent Manufacturing:NaOH is a key ingredient in the saponification process, where fats and oils are converted into soap. It also acts as a cleaning agent in various detergents and household cleaners.
Laboratory Applications
- pH Adjustment:NaOH is used to neutralize acidic solutions and adjust the pH of various solutions and buffers in laboratory settings.
- Precipitation Reactions:NaOH can precipitate metal ions from aqueous solutions, forming insoluble hydroxides. This property is utilized in analytical chemistry for qualitative and quantitative analysis of metals.
- Organic Synthesis:NaOH is employed as a base in various organic reactions, such as nucleophilic substitution and elimination reactions. It can also be used to generate alkoxides, which are important intermediates in organic synthesis.
Safety Considerations
NaOH is a highly corrosive substance that can cause severe burns and eye damage. It is important to take proper safety precautions when handling NaOH.
When working with NaOH, it is important to wear the following personal protective equipment (PPE):
- Goggles or safety glasses
- Rubber gloves
- Lab coat or apron
It is also important to work in a well-ventilated area and to avoid contact with skin and eyes. If NaOH comes into contact with skin or eyes, flush the affected area with water for at least 15 minutes and seek medical attention immediately.
Disposal Methods
NaOH should be disposed of properly. The best way to dispose of NaOH is to neutralize it with an acid, such as hydrochloric acid (HCl). The resulting solution can then be disposed of down the drain with plenty of water.
Upon adding NaOH to a 7.75, it’s worth noting that a similar legal dispute arose in the famous Krell v. Henry case , where a contract for the sale of goods was challenged due to a supervening impossibility. Returning to the chemical reaction, the addition of NaOH to a 7.75 solution can lead to significant changes in pH and other properties.
NaOH in Industrial Processes
NaOH is a highly versatile chemical with a wide range of industrial applications. It is primarily used in the production of paper, textiles, soaps, detergents, and various other chemicals. The industrial-scale production of NaOH is crucial to meet the high demand for this essential chemical.
Production Methods
NaOH is primarily produced through two main methods:
1. Electrolytic Process
This method involves passing an electric current through a brine solution (NaCl). The process results in the formation of NaOH, hydrogen gas, and chlorine gas.
2. Lime-Soda Process
This process involves treating limestone (CaCO3) with soda ash (Na2CO3) in a series of chemical reactions. The resulting product is NaOH, along with calcium carbonate (CaCO3) and carbon dioxide (CO2) as by-products.
Environmental Implications
The production of NaOH can have environmental implications, particularly when using the electrolytic process. This process generates chlorine gas, which can be harmful to the environment if not properly managed. Chlorine gas is a toxic and corrosive substance that can contribute to air pollution and respiratory problems.Therefore,
it is essential to implement proper measures to mitigate the environmental impact of NaOH production. These measures may include using efficient production technologies, recycling by-products, and implementing strict emission control standards to minimize the release of harmful substances into the environment.
Essential FAQs
What is the significance of the equivalence point in acid-base titrations?
The equivalence point marks the point at which the moles of acid and base are equal, resulting in complete neutralization. It allows us to determine the exact concentration of the unknown solution.
How does NaOH contribute to determining the equivalence point?
NaOH, as a strong base, reacts with the acid in a 1:1 ratio. By monitoring the pH changes during titration, we can identify the equivalence point when the pH reaches 7, indicating complete neutralization.
What are some common applications of NaOH in chemistry?
NaOH finds applications in pH adjustment, precipitation reactions, and organic synthesis. It is used in the production of soaps, detergents, paper, and textiles, among other industries.